Fatty acid β-oxidation (FAO) is the main bioenergy pathways in human prostate cancer (PCa) and promising new therapies vulnerabilities. Here we demonstrate the therapeutic efficacy of targeting FAO in prostate tumor clinical cultured ex vivo, and identify DECR1, encoding the enzyme rate-limiting for the acid oxidation of polyunsaturated fats (PUFAs), as excited expresse d in tissues of PCa and was associated with survival of relapse-free over short. DECR1 is androgen receptor (AR) gene targets of negative-regulated and, therefore, can promote PCa cell survival and resistance to therapy targeting AR.
DECR1 knockdown selectively inhibit β-oxidation of PUFAs, inhibits the proliferation and migration of PCa cells, including treatment-resistant line, and suppressed tumor cell proliferation and metastasis in xenograft mouse models. Mechanical, caused DECR1 targeting cellular accumulation of PUFA, enhanced mitochondrial oxidative stress and lipid peroxidation, and induced ferroptosis. These findings involves the oxidation of PUFAs through DECR1 as unexplored aspects of FAO that promotes PCa cell survival.
activation drastic changes in redox balance and the metabolism of platelets. Indeed, several signaling pathways have been shown to induce ROS production by NAPDH oxidase (NOX) and mitochondria, after activation of platelets. Platelet-derived ROS, in turn, further increases ROS production and consequent platelet activation, adhesion and recruitment in the loop auto-reinforcing.
This vicious circle results in platelet procoagulant phenotype and apoptosis, both accounting high thrombotic risk in oxidative stress-related illnesses. This review seeks to explain the molecular mechanisms underlying the production of ROS during platelet activation and altered redox balance effect on platelet function, with a focus on the major progress that has been made in redox biology of platelets. Furthermore, given the increased interest in this field, we also describe a method of up-to-date to detect platelets, ROS and bioenergy profile platelets, which have been proposed as potential biomarkers of disease.
Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis Both aerobic glycolysis and mitochondrial respiration necessary for osteoclast differentiation
Excessive bone resorption over bone formation is the root cause of bone loss that leads to osteoporosis fractures. Development of new antiresorptive therapy calls for a holistic understanding of osteoclast differentiation and function.
Although much has been learned about the regulation of the molecular biology of osteoclasts, little is known about the metabolism and bioenergetics requirements during osteoclastogenesis. Here, we report that glucose metabolism through oxidative phosphorylation (OXPHOS) is the dominant bioenergy pathways to support osteoclast differentiation. Meanwhile, increased production of lactate from glucose, which is known as aerobic glycolysis when oxygen is plentiful, it is also important for osteoclastogenesis.
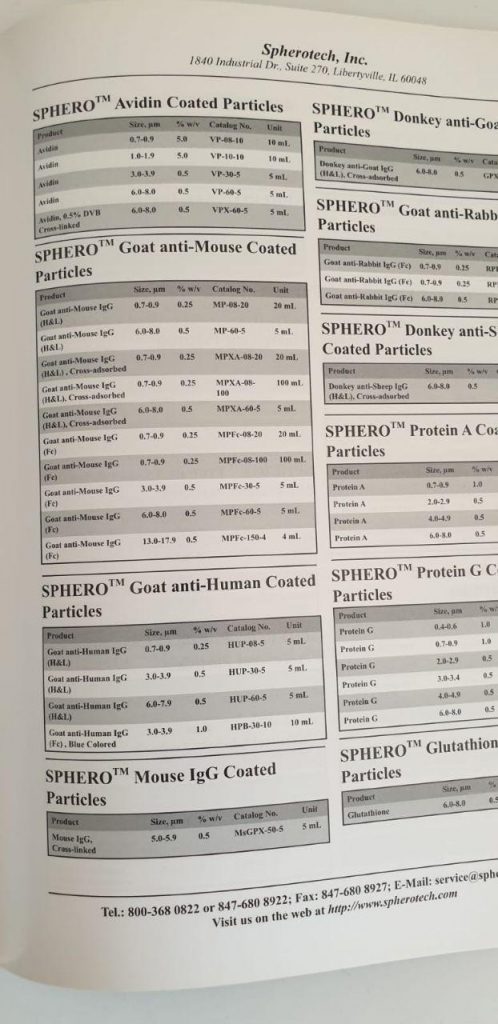
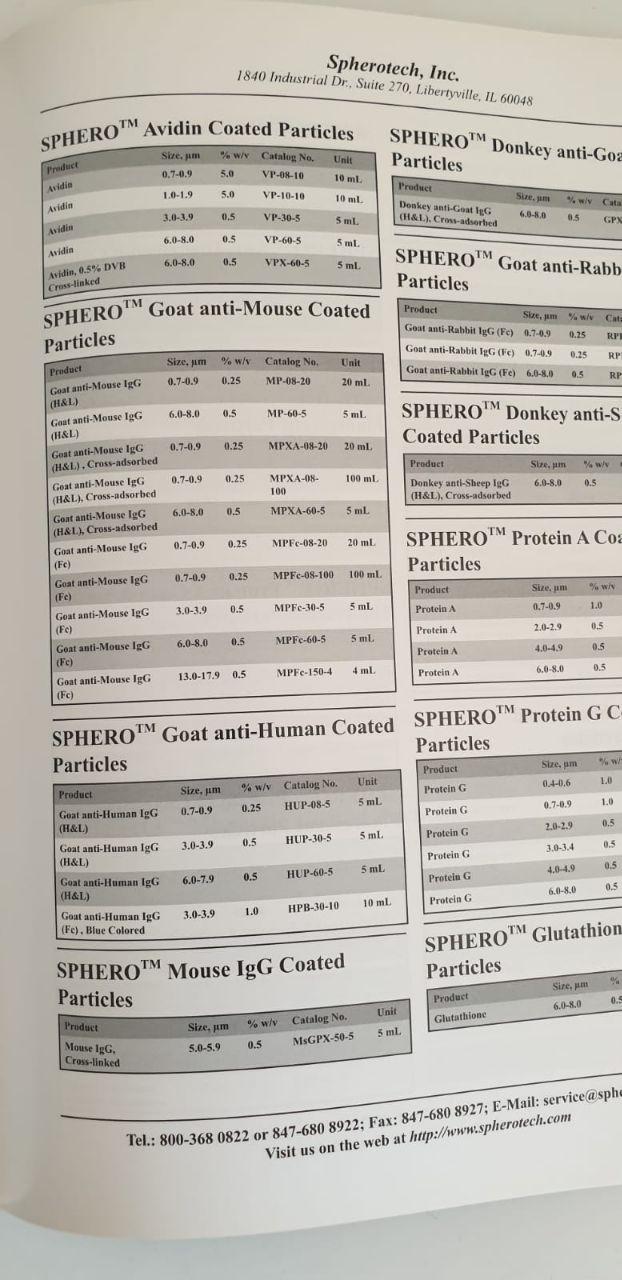
ExoPure? Mini Chromatography Columns
K1247-10
Biovision
each
EUR 874.8
ExoPure? Mini Chromatography Columns
K1247-20
Biovision
each
EUR 1214.4
ExoPure? Maxi Chromatography Columns
K1248-3
Biovision
each
EUR 1104
ExoPure? Maxi Chromatography Columns
K1248-6
Biovision
each
EUR 1690.8
ExoPure? Isolation Kit (Cell Media)
K1237-10
Biovision
each
EUR 796.8
ExoPure? Isolation Kit (Cell Media)
K1237-2
Biovision
each
EUR 470.4
ExoPure? Isolation Kit (Bio Fluids)
K1241-10
Biovision
each
EUR 836.4
ExoPure? Isolation Kit (Bio Fluids)
K1241-2
Biovision
each
EUR 483.6
ExoPure? Isolation Kit (Serum, Plasma)
K1238-10
Biovision
each
EUR 836.4
ExoPure? Isolation Kit (Serum, Plasma)
K1238-2
Biovision
each
EUR 470.4
ExoPure? Isolation Kit (Stem Cell Media)
K1239-10
Biovision
each
EUR 901.2
ExoPure? Isolation Kit (Stem Cell Media)
K1239-2
Biovision
each
EUR 510
ExoPure? Reagent (Overall Exosome Isolation, urine)
M1003-25
Biovision
each
EUR 992.4
ExoPure? Reagent (Overall Exosome Isolation, urine)
M1003-50
Biovision
each
EUR 1083.6
ExoPure? Reagent (Overall Exosome Isolation, cell media)
M1002-25
Biovision
each
EUR 992.4
ExoPure? Reagent (Overall Exosome Isolation, cell media)
M1002-50
Biovision
each
EUR 1083.6
ExoPure? Reagent (Overall Exosome Isolation, biological fluids)
M1001-10
Biovision
each
EUR 796.8
ExoPure? Reagent (Overall Exosome Isolation, biological fluids)
M1001-20
Biovision
each
EUR 1260
ExoPure? Reagent (Overall Exosome Isolation, biological fluids)
M1001-5
Biovision
each
EUR 633.6
ExoPure? Immunoplates (Overall Exosome Isolation, serum, colorimetric assay)
M1007-100-T
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, serum, luminometric assay)
M1008-100-W
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, serum, fluorimetric assay)
M1009-100-B
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, saliva, colorimetric assay)
M1010-100-T
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, saliva, luminometric assay)
M1011-100-W
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, saliva, fluorimetric assay)
M1012-100-B
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, saliva, colorimetric assay)
M1013-100-T
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, saliva, luminometric assay)
M1014-100-W
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, saliva, fluorimetric assay)
M1015-100-B
Biovision
each
EUR 888
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum)
M1038-10
Biovision
each
EUR 1005.6
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum)
M1038-20
Biovision
each
EUR 1546.8
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum)
M1038-3
Biovision
each
EUR 523.2
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum)
M1038-5
Biovision
each
EUR 796.8
ExoPure? Immunoplates (Overall Exosome Isolation, plasma, urine, colorimetric assay)
M1004-100-T
Biovision
each
EUR 1136.4
ExoPure? Immunoplates (Overall Exosome Isolation, plasma, urine, luminometric assay)
M1005-100-W
Biovision
each
EUR 888
ExoPure? Immunoplates (Overall Exosome Isolation, plasma, urine, fluorometric assay)
M1006-100-B
Biovision
each
EUR 888
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, cell media, 10 reactions)
M1030-10
Biovision
each
EUR 1005.6
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, cell media, 20 reactions)
M1030-20
Biovision
each
EUR 1546.8
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, cell media, 3 reactions)
M1030-3
Biovision
each
EUR 529.2
ExoPure? 0.4 micron Immunobeads (Overall Exosome Isolation, cell media, 5 reactions)
M1030-5
Biovision
each
EUR 796.8
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, cell media, 10 reactions)
M1031-10
Biovision
each
EUR 1005.6
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, cell media, 20 reactions)
M1031-20
Biovision
each
EUR 1546.8
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, cell media, 3 reactions)
M1031-3
Biovision
each
EUR 523.2
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, cell media, 5 reactions)
M1031-5
Biovision
each
EUR 796.8
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum, 10 reactions)
M1039-10
Biovision
each
EUR 1005.6
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum, 20 reactions)
M1039-20
Biovision
each
EUR 1546.8
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum, 3 reactions)
M1039-3
Biovision
each
EUR 523.2
ExoPure? 1.0 micron Immunobeads (Overall Exosome Isolation, plasma, urine, serum, 5 reactions)
M1039-5
Biovision
each
EUR 796.8
Exophilin 5 (EXPH5) Antibody
abx232909-100ug
Abbexa
100 ug
EUR 661.2
Exophilin 5 (EXPH5) Antibody
20-abx313705
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exophilin 5 (EXPH5) Antibody
abx232909-100g
Abbexa
100 µg
EUR 350
Exophilin 5 (EXPH5) Antibody
abx313705-100g
Abbexa
100 µg
EUR 362.5
Exophilin 5 (EXPH5) Antibody
abx313705-20g
Abbexa
20 µg
EUR 162.5
Exophilin 5 (EXPH5) Antibody
abx313705-50g
Abbexa
50 µg
EUR 250
Human Exophilin 5 (EXPH5)ELISA Kit
201-12-2684
SunredBio
96 tests
EUR 528
Description: A quantitative ELISA kit for measuring Human in samples from biological fluids.
Mouse Exophilin 5 (EXPH5) ELISA Kit
abx389223-96tests
Abbexa
96 tests
EUR 1093.2
Human Exophilin 5 (EXPH5) ELISA Kit
abx387228-96tests
Abbexa
96 tests
EUR 1093.2
Exophilin 5 (EXPH5) Antibody (HRP)
20-abx313706
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exophilin 5 (EXPH5) Antibody (HRP)
abx313706-100g
Abbexa
100 µg
EUR 362.5
Exophilin 5 (EXPH5) Antibody (HRP)
abx313706-20g
Abbexa
20 µg
EUR 162.5
Exophilin 5 (EXPH5) Antibody (HRP)
abx313706-50g
Abbexa
50 µg
EUR 250
EXO-Prep for exosome isolation from Urine
HBM-EXP-U25
HansaBioMed
30 ml (25 react)
EUR 234.36
Exophilin 5 (EXPH5) Antibody (FITC)
20-abx313707
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exophilin 5 (EXPH5) Antibody (FITC)
abx313707-100g
Abbexa
100 µg
EUR 362.5
Exophilin 5 (EXPH5) Antibody (FITC)
abx313707-20g
Abbexa
20 µg
EUR 162.5
Exophilin 5 (EXPH5) Antibody (FITC)
abx313707-50g
Abbexa
50 µg
EUR 250
EXO-Prep for exosome isolation from Cell Media
HBM-EXP-C25
HansaBioMed
25 ml (25 react)
EUR 234.36
Exophilin 5 (EXPH5) Antibody (Biotin)
20-abx313708
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exophilin 5 (EXPH5) Antibody (Biotin)
abx313708-100g
Abbexa
100 µg
EUR 362.5
Exophilin 5 (EXPH5) Antibody (Biotin)
abx313708-20g
Abbexa
20 µg
EUR 162.5
Exophilin 5 (EXPH5) Antibody (Biotin)
abx313708-50g
Abbexa
50 µg
EUR 250
Human Exophilin 5, EXPH5 GENLISA ELISA
KBH2683
Krishgen
1 x 96 wells
EUR 286
EXO-Prep for exosome isolation from Plasma and serum
HBM-EXP-B5
HansaBioMed
5 ml
EUR 181.44
Exph5 ELISA Kit| Mouse Exophilin-5 ELISA Kit
EF014853
Lifescience Market
96 Tests
EUR 826.8
Exopolyphosphatase (PPX1) Antibody
20-abx300871
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exopolyphosphatase (PPX1) Antibody
abx300871-100g
Abbexa
100 µg
EUR 362.5
Exopolyphosphatase (PPX1) Antibody
abx300871-20g
Abbexa
20 µg
EUR 162.5
Exopolyphosphatase (PPX1) Antibody
abx300871-50g
Abbexa
50 µg
EUR 250
Exopolyphosphatase (PPX1) Antibody (HRP)
20-abx300912
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exopolyphosphatase (PPX1) Antibody (HRP)
abx300912-100g
Abbexa
100 µg
EUR 362.5
Exopolyphosphatase (PPX1) Antibody (HRP)
abx300912-20g
Abbexa
20 µg
EUR 162.5
Exopolyphosphatase (PPX1) Antibody (HRP)
abx300912-50g
Abbexa
50 µg
EUR 250
Exopolyphosphatase (PPX1) Antibody (FITC)
20-abx300913
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Exopolyphosphatase (PPX1) Antibody (FITC)
abx300913-100g
Abbexa
100 µg
EUR 362.5
Exopolyphosphatase (PPX1) Antibody (FITC)
abx300913-20g
Abbexa
20 µg
EUR 162.5
Exopolyphosphatase (PPX1) Antibody (FITC)
abx300913-50g
Abbexa
50 µg
EUR 250
Exopolyphosphatase (PPX1) Antibody (Biotin)
20-abx300914
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Escherichia coli O6:H1 Exopolyphosphatase (ppx)
1-CSB-YP360285EGX
Cusabio
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100ug 10ug 1MG 200ug 500ug 50ug
Description: Recombinant Escherichia coli O6:H1 Exopolyphosphatase(ppx) expressed in Yeast
Escherichia coli O6:H1 Exopolyphosphatase (ppx)
1-CSB-EP360285EGX
Cusabio
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100ug 10ug 1MG 200ug 500ug 50ug
Description: Recombinant Escherichia coli O6:H1 Exopolyphosphatase(ppx) expressed in E.coli
Escherichia coli Exopolyphosphatase (ppx)
1-CSB-EP365319ENV
Cusabio
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100ug 10ug 1MG 200ug 500ug 50ug
Description: Recombinant Escherichia coli Exopolyphosphatase(ppx) expressed in E.coli
Exopolyphosphatase (PPX1) Antibody (Biotin)
abx300914-100g
Abbexa
100 µg
EUR 362.5
Exopolyphosphatase (PPX1) Antibody (Biotin)
abx300914-20g
Abbexa
20 µg
EUR 162.5
Exopolyphosphatase (PPX1) Antibody (Biotin)
abx300914-50g
Abbexa
50 µg
EUR 250
Prune Exopolyphosphatase 1 (PRUNE1) Antibody
20-abx334087
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Prune Exopolyphosphatase 1 (PRUNE1) Antibody
abx334087-100g
Abbexa
100 µg
EUR 362.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody
abx334087-20g
Abbexa
20 µg
EUR 162.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody
abx334087-50g
Abbexa
50 µg
EUR 250
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (HRP)
20-abx337306
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (HRP)
abx337306-100g
Abbexa
100 µg
EUR 362.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (HRP)
abx337306-20g
Abbexa
20 µg
EUR 162.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (HRP)
abx337306-50g
Abbexa
50 µg
EUR 250
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (FITC)
20-abx337307
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (FITC)
abx337307-100g
Abbexa
100 µg
EUR 362.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (FITC)
abx337307-20g
Abbexa
20 µg
EUR 162.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (FITC)
abx337307-50g
Abbexa
50 µg
EUR 250
Bovine PRUNE1 / Exopolyphosphatase PRUNE1 ELISA Kit
E6149b
EIAab
96T
EUR 900
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (Biotin)
20-abx337308
Abbexa
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100 ug 1 mg 200 ug 20 ug 50 ug
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (Biotin)
abx337308-100g
Abbexa
100 µg
EUR 362.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (Biotin)
abx337308-20g
Abbexa
20 µg
EUR 162.5
Prune Exopolyphosphatase 1 (PRUNE1) Antibody (Biotin)
abx337308-50g
Abbexa
50 µg
EUR 250
Saccharomyces cerevisiae Exopolyphosphatase (PPX1)
1-CSB-YP334459SVG
Cusabio
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
Ask for price
100ug 10ug 1MG 200ug 500ug 50ug
Description: Recombinant Saccharomyces cerevisiae Exopolyphosphatase(PPX1) expressed in Yeast
Recombinant Escherichia coli O6:H1 Exopolyphosphatase (ppx)
CSB-YP360285EGX
Cusabio
6366 mg
Ask for price
Recombinant Escherichia coli Exopolyphosphatase(ppx)
AP76627
SAB
each
Ask for price
Recombinant Escherichia coli Exopolyphosphatase(ppx)
AP74215
SAB
1mg
EUR 2826
Genetic deletion in osteoclast progenitor Glut1 reduce aerobic glycolysis without sacrificing OXPHOS, but nonetheless reduced osteoclast differentiation in vitro. The progenitor Glut1 deficiency leads to osteopetrosis because fewer osteoclasts specialized in female rats. Thus, Glut1-mediated glucose metabolism through the production of lactic and OXPHOS both required for normal osteoclastogenesis.